Recently, a group of researchers led by Dr. Honglin Jia from Harbin Veterinary Research Institute (HVRI) of Chinese Academy of Agricultural Sciences (CAAS), collaborating with Dr. Noboru Mizushima of Tokyo University and other researchers found an evolution from covalent conjugation to non-covalent interaction in the ubiquitin-like ATG12 system. This finding suggests that ubiquitin-like covalent conjugation can evolve to a simpler non-covalent interaction, most probably when the system has a limited number of targets. The article was published in “Nature Structural & Molecular Biology (NSMB-A40974B )”.
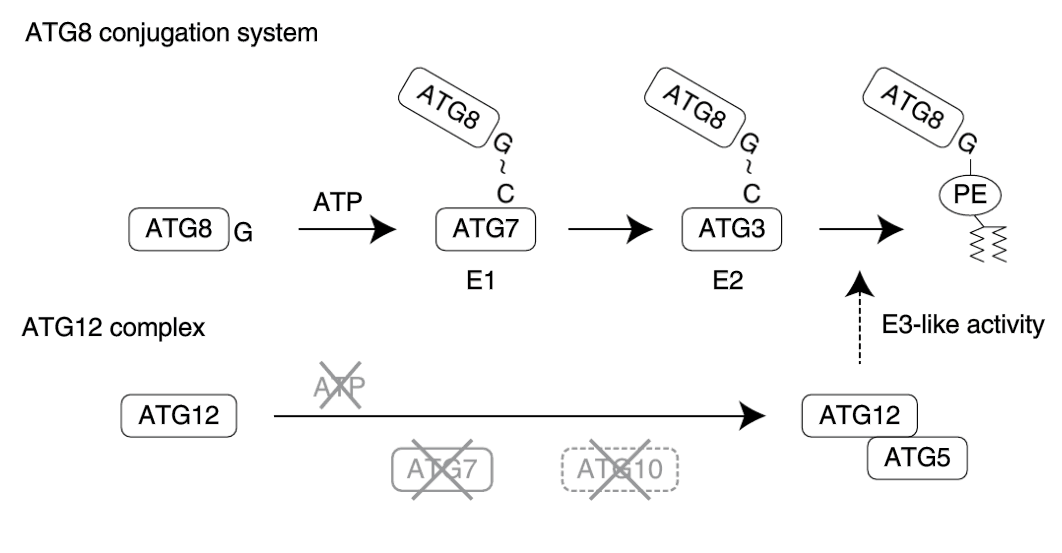
Ubiquitin and Ubiquitin like proteins (UBLs) are small proteins that modify various substrates and mediate important cellular processes such as proteasomal and autophagic degradation, DNA repair, cellular signaling, and immune responses in plant and mammalian hosts. These UBLs are covalently linked to substrate proteins via an isopeptide bond with target proteins. Ubiquitin conjugation is an ATP-dependent reaction that is catalyzed by sequential actions of ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin ligase (E3). Ubiquitin-like systems also have their own catalyzing enzymes that create an isopeptide bond between a UBL and its target. One of the major purposes of such energetically expensive covalent linkages is to bind UBLs with multiple substrates that do not necessarily have cognate interfaces. Exceptions are now uncovered: in Plasmodium and Toxoplasma species and in yeast Komagataella phaffii, ATG12 and ATG5 form a non-covalent yet functional complex. Thus, any ubiquitin-like system that has one or a limited number of substrates may no longer need to form covalent linkages.
This study was supported by National Key Research and Development Program of China (No. 2017YFD0500400). More details are available on the link below: https://rdcu.be/bsQRF
|
